Is C10h20 an Alkane or Alkene
The following is a list of the first ten Alkenes. C6H10 C10H20 C7H16 C8H14.

Alkanes Vs Alkenes Vs Alkynes Chemistry Lessons Organic Chemistry Study Chemistry Basics
The root and endings are the same.
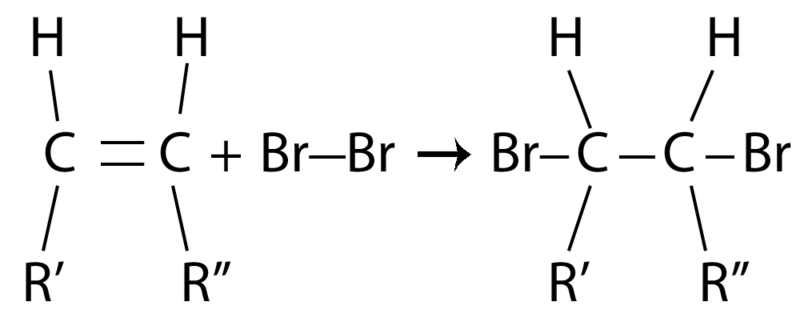
. Pentenes Pent-1-ene EPent-2-ene ZPent-2-ene Methylbutenes 2-Methylbut-1-ene 3-Methylbut-1-ene 2-Methylbut-2. Compound A C10H22O undergoes reaction with dilute. Since alkenes are hydrocarbons they burn in oxygen to give water and Carbon dioxide.
Branched alkenes and Isomerism. Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula CnH2n2. What is the structure of 2.
26 rows Alkane Names Functional Groups. 1-decene is a colorless watery liquid with a pleasant odor. Alkane Names Primary Secondary Tertiary Quaternary Carbons Common Functional Groups.
C18H40 QUESTION 2 The fuel identified. The carbons with appropriate. The molecular formula C10H20 molar mass.
14026 gmol exact mass. The combustion of alkanes and other hydrocarbons obtained from fossil fuels adds a tremendous amount of CO2 to the atmosphere each year. C 2 H 4 3O 2 2CO 2 2H 2 O.
Predict the molecular formula of decane and explain your. C-H and C-C Bonds. Decene is an alkene with the formula C10H20.
Name the alkene with molecular formula C10H20 Kerala Med 2003 Dodecene. Alkenes have at least one double. Ethene C2H4 Propene C3H6 Butene C4H8 Pentene C5H10 Hexene C6H12 Heptene C7H14 Octene C8H16.
Alkanes are solid liquid or gas at room temperature. There are many isomers of decene depending on the position and geometry. Based on the molecular formula determine whether each compound is an alkane alkene or alkyne.
However if this is a cyclic alkane you would have C10H20 cyclodecane. Alkenes have a double bond. 1401565 u may refer to.
Answer 1 of 3. Based on the molecular formula determine. Decene contains a chain of ten carbon atoms with one double bond.
Its molecules contain 10 carbon atoms. A large and structurally simple class of hydrocarbons includes those substances in which all the carbon-carbon bonds are single bonds. The prefix cyclo- is used for alkane and alkene hydrocarbon ring compounds.
View Homework Help - Identify the alkane from CH 1100 at Indiana Institute of Technology. Index of chemical compounds with the same. Alkenes are unsaturated hydrocarbons because they have a double C-C bond.
Up to 24 cash back Alkenes. Chemistry questions and answers. There are also numerous options for a branching alkane with various names and carbon to hydrogen.
CnH2n2 where n is the number of carbon atoms in the molecule Decane is an alkane. Here are the six possible alkene isomers of C_5H_10. Common alkanes include methane natural gas propane heating and cooking fuel butane lighter fluid and octane automobile fuel.
1-decene is an alkene that is decane containing one double bond at position 1. These are called saturated hydrocarbons. USCG 1999 CAMEO Chemicals.
A brief structure shows only the bonds.
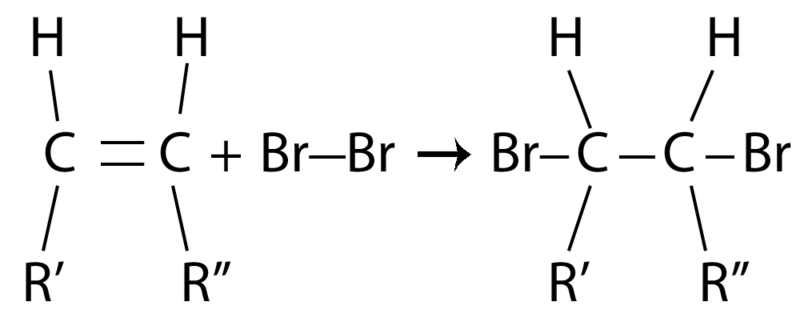
Alkanes Alkenes And Alkynes Vce Chemistry
What Is The Difference Between The Alkanes And The Alkenes Quora
What Is The Difference Between The Alkanes And The Alkenes Quora
No comments for "Is C10h20 an Alkane or Alkene"
Post a Comment